Abstract
Background: ME-401, a potent and selective oral PI3kδ inhibitor, achieved a high rate of early and durable responses in patients with follicular lymphoma (FL), chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) when administered once daily in 28-day cycles on a continuous schedule (CS) in a dose escalation Phase 1b study (Soumerai et al, ASCO 2018:#7519). The most common adverse events (AEs) were the delayed onset (beyond Cycle 2) of grade 3 diarrhea and rash, which were reversible with drug interruption and/or corticosteroids. These delayed AEs were thought to be due to pathway inhibition in regulatory T cells (Treg) leading to a disruption in immune homeostasis. We hypothesized that an intermittent schedule (IS) beyond Cycle 2 might mitigate or reduce the incidence of significant delayed AEs. The IS tested was selected based on the kinetics of Treg repopulation, and consists of ME-401 administered on days 1-7 of a 28-day cycle. We report preliminary results of this strategy.
Methods: Group 1 included 31 patients with relapsed FL (n = 22) or CLL/SLL (n = 9) who received ME-401 on a CS at doses ≥60 mg per day. 11/29 patients (38%) who received >2 cycles of therapy had developed delayed grade 3 AEs on CS and could be re-challenged with either the CS or IS (from December 2017 onward) following recovery from toxicity. The other 18/29 patients (62%) had not developed a grade 3 AEs of interest on CS and, beginning in December 2017, were switched to IS after a median of 26 weeks (range, 8-49) of daily dosing. Group 2 included 15 patients with relapsed FL (n = 9), diffuse large B-cell lymphoma (n = 4), marginal zone lymphoma (MZL, n = 1), and CLL (n = 1) who received rituximab 375 mg/m2 x 8 doses over 6 months and ME-401 at 60 mg daily x 2 cycles then switched to the IS. Group 3 includes 30 patients with relapsed FL/CLL/SLL enrolling in an expansion cohort of ME-401 alone at 60 mg daily x 2 cycles then switching to IS.
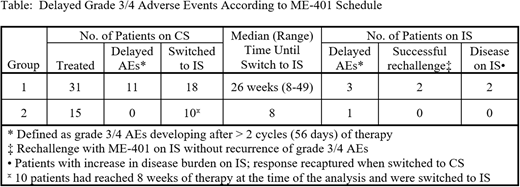
Results: Group 1: Of the 11 patients who developed a delayed grade 3 AE on CS, 6 were never re-challenged, 2 were re-challenged with CS with recurrence of their AE, and 3 were re-challenged with IS without recurrence of their AE. Of the 18 patients switched to the IS, and with a median follow-up of 5.2 months (range, 2.3-6.6) on IS, 3 developed grade 3 diarrhea on IS, 2 in the first cycle and 1 in the second cycle after the switch to IS, of whom 2 have been re-treated with IS for 1+ and 5+ months without recurrence of the AE. One patient was not evaluable for response due to discontinuation on Day 28 for personal reasons and 27/30 (90%) evaluable patients achieved an objective response. With a median follow-up of 9.4 months (range, 2.2-17.5) from enrollment, only 2/27 (7%) responders had disease progression (PD) on CS and were discontinued. Of the 18 patients who were switched to IS, only 1 SLL patient with a partial response (PR) achieved on CS developed PD on IS and was successfully rescued with switch back to CS. Another CLL patient in PR on CS had 10% increase in SPD from nadir in Cycle 5 on the IS and was switched back to CS.
Group 2: 10/15 patients have completed 2 cycles of daily dosing at the time of analysis and were systematically switched to IS. With a median follow-up of 3.4 months (range, 1.5-5.7) on IS, only 1/10 patients developed delayed grade 3 diarrhea in the first cycle after switch to IS. 7/10 patients (70%) with FL/MZL achieved an objective response and no PD was reported with a median follow-up of 5.2 months (range, 3.1-7.5) from enrollment.
Conclusions: Preliminary data suggest that switching to an intermittent schedule consisting of ME-401 administered on days 1-7 of a 28-day cycle following 2 cycles of continuous daily dosing was associated with a low rate of delayed grade 3 AEs and was associated with preservation of response in the vast majority of patients. All delayed grade 3 AEs of interest on IS occurred within 1-2 cycles of switching from CS to IS, suggesting that these might have represented a delayed effect of daily dosing. IS may also be a suitable re-treatment strategy in patients with delayed AEs on CS. Safety and efficacy data for the expansion cohort of 30 patients treated with ME-401 at 60 mg for 2 cycles then switched to IS will be presented at the meeting. A randomized study comparing CS and IS in FL is planned.
Zelenetz:Abbvie: Research Funding; Celgene: Consultancy; AstraZeneca: Consultancy; Novartis/Sandoz: Consultancy; Amgen: Consultancy; Gilead: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding. Reddy:MEI Pharma: Research Funding. Stathis:Oncology Therapeutic Development: Research Funding. Ghalie:MEI Pharma: Employment, Equity Ownership; Viracta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Pagel:Pharmacyclics, an AbbVie Company: Consultancy; Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal